Valvular Heart Disease
Guideline Alert 2007: AHA Prevention of Infective Endocarditis (IE)
(Circulation. 2007;116;1736-1754.)
The AHA has issued its first revision of SBE (IE) prophylaxis guidelines in 10 years.
Primary Reasons for Revision of the SBE (IE) Prophylaxis Guidelines:
- IE is much more likely to result from frequent exposure to random bacteremia associated with daily activities than from bacteremia caused by a dental, GI tract, or GU tract procedure.
- Prophylaxis may prevent an exceedingly small number of cases of IE, if any, in individuals who undergo a dental, GI tract, or GU tract procedure.
- The risk of antibiotic-associated adverse events exceeds the benefit, if any, from prophylactic antibiotic therapy.
- Maintenance of optimal oral health and hygiene may reduce the incidence of bacteremia from daily activities and is more important than prophylactic antibiotics for a dental procedure to reduce the risk of IE.
Cardiac Conditions for Which SBE (IE) Prophylaxis For Dental Procedures Is Still Indicated:
- Prosthetic cardiac valve
- Previous IE
- Congenital heart disease (CHD)
- Unrepaired cyanotic CHD, including palliative shunts and conduits
- Completely repaired congenital heart defect with prosthetic material or device, whether placed by surgery or by catheter intervention, during the first 6 months after the procedure†
- Repaired CHD with residual defects at the site or adjacent to the site of a prosthetic patch or prosthetic device (which inhibit endothelialization)
- Cardiac transplantation recipients who develop cardiac valvulopathy
Cardiac Conditions for Which SBE (IE) Prophylaxis for Dental Procedures Is No Longer Indicated:
- *Except for the conditions listed above, antibiotic prophylaxis is no longer recommended for any other form of CHD (Bicuspid AV, ASD, VSD, PDA, pulmonary stenosis).
- Acquired valvular heart disease (AS, MS, AR, MR)
- MVP with or without murmur
- HOCM with inducible or resting gradient
Procedures for Which SBE (IE) Prophylaxis Recommended (for Conditions Still Indicated above):
- All dental procedures that involve manipulation of gingival tissue or the periapical region of teeth or perforation of the oral mucosa
*The following procedures and events do not need prophylaxis: routine anesthetic injections through non-infected tissue, taking dental radiographs, placement of removable prosthodontic or orthodontic appliances, adjustment of orthodontic appliances, placement of orthodontic brackets, shedding of deciduous teeth, and bleeding from trauma to the lips or oral mucosa.
- Invasive procedure of the respiratory tract that involves incision or biopsy of the respiratory mucosa, such as tonsillectomy and adenoidectomy.
- Invasive respiratory tract procedure to treat an established infection, such as drainage of an abscess or empyema (cover for strep viridans or if identified pathogen such as staph aureus cover with appropriate antibiotic
- Surgical procedure that involves infected skin, skin structure, or musculoskeletal tissue, it is reasonable that the therapeutic regimen administered for treatment of the infection contain an agent active against staphylococci and β - hemolytic streptococci, such as an anti-staphylococcal penicillin or a cephalosporin, vancomycin or clindamycin may be administered to patients unable to tolerate a β -lactam or who are known or suspected to have an infection caused by a methicillin-resistant strain of staphylococcus.
Procedures for Which SBE (IE) Prophylaxis No Longer Recommended:
GI or GU procedures:
- Endoscopy
- Colonoscopy
- Elective cystoscopy or urinary tract manipulation*
Regimen Recommendations for Dental Procedures: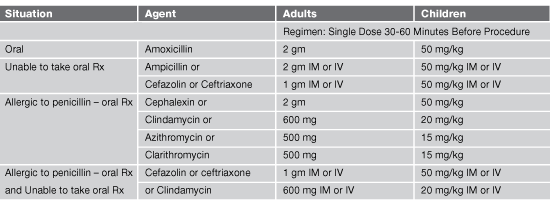
Commentary: These guideline revisions change longstanding practices and beliefs by reducing the need for SBE prophylaxis for many conditions and indications previously considered essential. There will likely be some reluctance to change clinical practices so dramatically and so abruptly. Although evidence indicates that the risk of developing IE from a dental procedure in the general population is in the order of
1 in 14 million, with MVP - 1in 1.1 million, with CHD (congenital heart disease) - 1 in 475 thousand, with RHD - 1in 142 thousand, with prosthetic cardiac valves - 1in 114 thousand and with prior IE - 1in 95 thousand dental procedures, many physicians and patients will be reluctant to change established practice. Changes to the guidelines are to emphasize IE prophylaxis in only those patients with the highest risk of adverse outcome from IE. For all others the benefit of IE prophylaxis is unproven and the risk of antibiotic side effects potentially outweighs the potential reduction in IE occurrence. Regardless, guidelines are only guidelines, and should always be applied in the context of best clinical judgment and experience.
|